

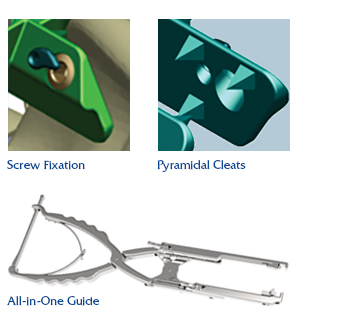
The Reli™ SP PLUS Spinous Plating System is the first spinous plating system to receive 510(k) marketing clearance from the U.S. Food and Drug Administration (FDA) for utilization with supplemental screw fixation. The two-plate design features an unprecedented fixation option that permits surgeons to attach the plates to the spinous process with screws, in addition to cleats, creating a more rigid construct, reducing the occurrence of occult spinous process fracture and mitigating against dislocation migration.
System features:
-
First to receive FDA clearance for use with supplemental screw fixation
- Supplemental Screw Fixation and Pyramidal Cleats create a more rigid construct
- Reduces occurrence of occult spinous process fracture
-
Mitigates against dislocation migration
-
Four Plate Sizes for optimal anatomical fit
For additional information, please contact:
Phone: 877-780-4370 Fax: 601-420-5501

